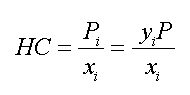
HenryConstant_Water
The HenryConstant_Water function returns Henry's Law constant for a specified fluid in a water solvent. Henry's Law can be defined in different ways. Here, the Henry's Law constant is defined as:
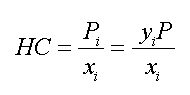
where
HC is the Henry's Law constant returned by this function with units of pressure. The pressure units are as set in EES with $UnitSystem directive or the Preferences.
P_i is the partial pressure of component i in the gas above water
y_i is the mole fraction of component i in the gas above the water
P is the total pressure
x_i is the mole fraction of component i in the liquid at the interface with the gas phase.
The function accepts one argument in addition to the name of the fluid and that must be temperature in the units set within EES.
Henry's Law constant information is provided for most of the pure fluids in the real-fluids data base. The information is from:
R. Sander
Compilation of Henry's law constants (version 4.0) for water as solvent
Atmos. Chem. Phys., 15, 4399-4981, 2015 Website
Example:
$UnitSystem SI K kPa mass
T=300 [K]
HC=henryconstant_water(CarbonDioxide,T=T)
{Solution:
HC=176194 [kPa]
}